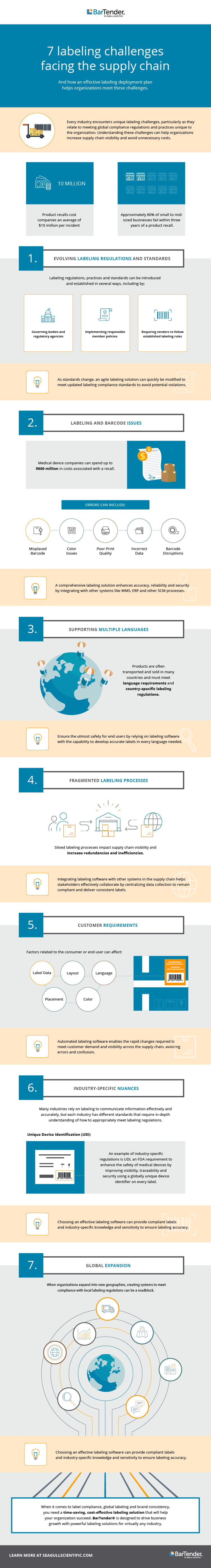
Every industry encounters unique barcode issues and labeling challenges, particularly as they relate to meeting global compliance regulations and practices unique to the organization. When enterprise organizations need to meet these evolving standards, it’s crucial they have the right technology to maintain supply chain traceability and transparency and efficiency.
Unfortunately, if an organization relies on a complicated labeling system, it may experience inefficiencies that incur unnecessary costs related to inconsistent branding, labeling compliance and the inability to properly scale and grow. When organizations have labeling issues like product recalls or errors, they can experience expensive chargebacks and penalties.
The consulting firm Deloitte estimates that in the US, product recalls cost companies an average of $10 million dollars per incident. And approximately 80 percent of small to mid-sized businesses fail within three years of a product recall — you can attribute this to the loss of sales, reputation and brand value.
But understanding these challenges, and how an effective labeling deployment plan meets these challenges, can help organizations increase supply chain visibility and avoid unnecessary costs.
Read on to learn about seven common labeling challenges and how to solve them with a compliant, effective labeling solution.
1. Evolving labeling regulations and standards
One of the biggest challenges that enterprise organizations face with global labeling is compliance. The frequency with which regulations change can make it difficult to keep up with and manage the immense variability in labeling for a global supply chain.
Labeling regulations, practices and standards can be introduced and established in several ways, including:
- By governing bodies and regulatory agencies
- Industry associations who want to get ahead of regulatory agencies by implementing responsible member policies. Members of these associations show their commitment to quality and performance, and thus, regulators respect their industry-specific guidelines. Examples include Responsible Distribution for the National Association of Chemical Distributors and the Produce Traceability Initiative (PTI), which implemented “a standardized industry approach to enhance the speed and efficiency of traceability systems” in the food industry.
- Large retailers like Walmart, Costco and Amazon require vendors to follow certain labeling rules that they establish. For example, when the PTI came out, it was not widely considered until Walmart stated they would only accept produce with the PTI label (enabled by GS1 — 128 barcode). That labeling requirement is now known as the Walmart produce label and is the industry standard seen at both large and small grocers.
For industries such as aerospace, medical device, pharmaceutical and food and beverage, the challenges of managing labeling regulations are amplified by the stringent requirements from various governing bodies, including:
- EU Food Allergy Labeling (FIC)
- FDA 21 CFR 11
- GHS
- UDI
- GS1 Digital Link
- EU MDR
- FSMA
- DSCSA
- EU FMD
As standards change, an agile labeling solution can quickly be modified to meet updated labeling compliance standards to avoid potential violations or barcode issues that could be costly. Managing, securing and controlling label printing for a global supply chain is made possible by labeling software that easily integrates with multiple devices and other systems and processes.
2. Labeling and barcode issues
Labeling errors affect more than just the end user but can disrupt the entire supply chain by providing incorrect information to each stakeholder along the way. Additionally, labeling errors can lead to product recalls and supply chain inefficiencies, which can result in extra costs for relabeling and stall further innovation planned by the organization.
Errors can include:
- Incorrect data: Errors with symbology, date, ingredients, component or order or information can affect labels and lead to recall.
- Misplaced barcode: If a barcode is on a curve or doesn’t have proper light areas around the barcode, it may not be readable.
- Color issues: High contrast increases readability, and low contrast decreases readability. GS1 US standards have standard color and size codes to help organizations maintain compliance and readability.
- Poor print quality: Barcode scanning can be ineffective if barcode lines aren’t properly spaced or additional ink spots are detected.
- Barcode disruptions: Labels that are covered, peeling, torn or folded may be rendered unreadable.
Recalls can be extremely costly for enterprise organizations. A 2017 McKinsey Report found that medical device companies can spend up to $600 million in costs associated with a recall. While product recalls can be related to more than just labels, it’s crucial for organizations to print and use accurate labels to avoid such costs.
A comprehensive labeling solution will enhance accuracy, reliability and security by integrating with other systems like warehouse management systems (WMS), enterprise resource planning (ERP) and other supply chain management (SCM) processes. Sourcing data from these other systems can help avoid duplication, streamline the process of label creation and ensure traceability in the event of a product recall.
3. Supporting multiple languages
Global organizations who operate in multiple countries are tasked with the additional challenge of localization, or creating labels unique to a specific geographic region and language. This challenge also comes into play if an organization is looking to expand into a new market.
Products from food to medical devices are often transported and sold in many countries, and thus, must meet language requirements in addition to country-specific labeling regulations. Without proper translation support on labels, organizations could be left with recalls or brand distrust.
To ensure the utmost safety for end users, it’s important for organizations to rely on labeling software with the capability to develop accurate labels in every language needed. Features like auto translation and the ability to accommodate modern writing systems can replace outdated, manual processes that can be costly and time consuming.
4. Fragmented labeling processes
The labeling process is an intricate one that involves numerous stakeholders in the supply chain. It’s also a process that encounters ongoing changes in regulations, scaling or downsizing, mergers, economic fluctuations, expanding into new markets, new products and rebranding. With so many considerations, it’s easy for the labeling process to get confusing.
When labeling processes get siloed, and supply chain visibility is limited, redundancies and inefficiencies are common. This can lead to increased costs due to poor processes and errors that necessitate relabeling.
Consider a medical device company that is entering a new market in another country. This company will face global labeling challenges as they work to meet the demands of localization and regulations for that specific location across their supply chain. Without proper visibility, the chance for errors and miscommunications is higher.
Integrating enterprise labeling software with other systems in the supply chain helps stakeholders effectively collaborate by centralizing data collection to remain compliant and deliver consistent labels no matter where they’re being printed. Additionally, dynamic labeling offers the flexibility to pivot when there are changes in standards, formats, colors, products and language.
5. Customer requirements
Factors related to the consumer or end user can affect label data, layout, language and placement, even impacting what colors are used. Customers are also more demanding about the level of transparency in labeling, wanting to know as much information as possible about the product they’re using or consuming.
Without a dynamic labeling system in place, labeling and barcode issues can impact production. It’s difficult for enterprise organizations to make the changes necessary to meet every end-user if they don’t have a centralized system for collecting data and making changes.
Automated labeling software enables the rapid changes required to meet customer demand and visibility across the supply chain, avoiding errors and confusion. This not only saves time and money for an organization but helps support brand credibility and improves customer satisfaction.
6. Industry-specific nuances
Many industries rely on labeling to communicate information effectively and accurately, but each industry has different standards that require in-depth understanding of how to appropriately meet labeling regulations.
Even if an organization is using labeling software, if it’s not up to date with the correct industry information or regulatory standards for that industry, it can be a challenge for organizations to remain consistent and accurate.
An example of industry-specific regulations is Unique Device Identification (UDI), which is a regulatory requirement to enhance the safety of medical devices by improving visibility, traceability and security using a globally unique device identifier on every device. Without this standard, it would be challenging for medical device manufacturers to keep data consistent.
Choosing an effective labeling software for enterprise businesses can provide compliant labels and industry-specific knowledge and sensitivity to ensure labeling accuracy. Using the UDI example, the right labeling solution will offer:
- Global change management
- Centralized, configurable role-based access for modification, setup and printing
- Audit trails to fulfill regulatory standards
7. Global expansion
When organizations expand into new geographies, creating systems to meet compliance with local labeling regulations can be a roadblock.
These challenges are exponential for industries such as medical device or automotive manufacturing who engage in component manufacturing. First- and second-tier suppliers to these organizations can be located around the world. Standardizing processes can be difficult without a good labeling solution.
When organizations are ready to scale and grow into new markets, implementing enterprise labeling software solutions will allow systems to integrate across the supply chain. So, a warehouse management system (WMS) operated in the U.S., for example, can seamlessly integrate labeling with a manufacturer in Japan. As products are transported and packaged, labeling never gets compromised.
The ability to integrate with Enterprise Resource Planning (ERP) systems is an important consideration when choosing the right barcode software. ERPs like Oracle, SAP, Infor, IBM, Sage, HighJump, Microsoft Dynamics, WebSphere and Epicor can be integrated with labeling software so there's never a gap between label development and printing. But very few barcode and labeling solutions can integrate data from any source.
Overcome labeling challenges with BarTender
When it comes to label compliance, global labeling and brand consistency, you need a time-saving, cost-effective labeling solution that will help your organization succeed.
BarTender® is designed to drive business growth with powerful labeling solutions for virtually any industry, including aerospace, pharmaceutical, medical device, chemical and food and beverage. Whether you need labeling software for a small business or a centralized system for a global organization, BarTender has the ability to meet global regulations with efficiency and growth in mind.
Learn more by reading about how to choose labeling software or contact us to speak with a representative about our comprehensive labeling solutions.